Fitness
Oral Tranexamic Acid Boosts Melasma Outcomes as Added Rx
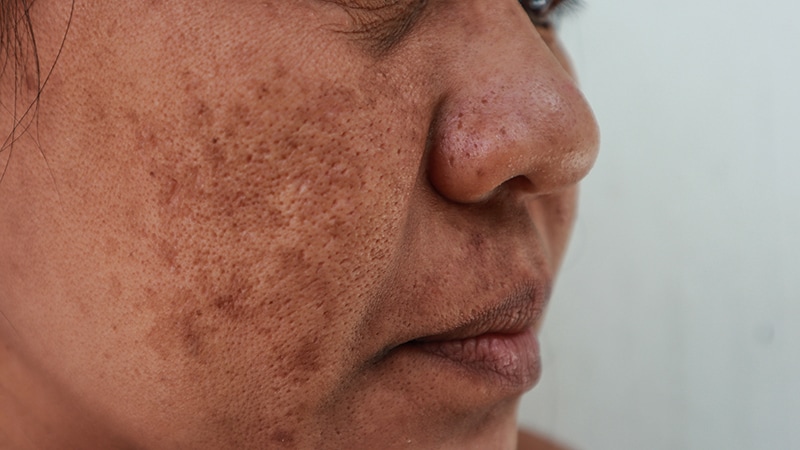
TOPLINE:
A meta-analysis showed that the use of oral tranexamic acid along with the standard triple combination cream (TCC) reduces melasma severity and recurrence in patients with melasma, without increasing toxicity.
METHODOLOGY:
- Current treatments for melasma focus on inducing remission and preventing relapse. Tranexamic acid, an antifibrinolytic drug, has shown promise in recent studies, but its optimal use, either alone or as an adjunct to TCC, remains unclear.
- Researchers conducted a meta-analysis of four randomized controlled trials patients that compared oral tranexamic acid plus TCC (hydroquinone, retinoic acid, and hydrocortisone) and TCC alone in 480 patients with melasma, divided almost evenly into the two treatment groups.
- The main outcome was the change in the Melasma Severity Area Index (MASI) score and recurrence rate from baseline.
TAKEAWAY:
- Patients treated with oral tranexamic acid plus TCC showed a greater reduction in MASI scores compared with those who received TCC alone (mean difference, −3.10; P = .03).
- The recurrence rate of melasma was significantly lower in the tranexamic acid plus TCC group (risk ratio [RR], 0.28; P
- There was no significant difference in the incidences of erythema (RR, 0.63; P = .147) and burning (RR, 0.59; P = .131).
IN PRACTICE:
“Evidence indicates that oral tranexamic acid confers clinical benefits, contributing to the enhancement of treatment outcomes in melasma when used in conjunction with TCC therapy,” and results are promising with regards to minimizing recurrence, the authors concluded.
SOURCE:
The study was led by Ocílio Ribeiro Gonçalves, MS, of the Federal University of Piauí, Teresina, Brazil, and was published online on June 8, 2024, in Clinical and Experimental Dermatology.
LIMITATIONS:
There was heterogeneity across studies, including different methods of administration, treatment protocols (including dosage), and timing of treatment.
DISCLOSURES:
The study reported receiving no funding. The authors declared no conflicts of interest.


)






